本帖最后由 costa_na 于 2013-11-28 12:50 编辑
6楼的翻译 - 第一部分
MO21.10 SERIAL MONITORING OF PLASMA EGFR T790M LEVELS AND EVALUATION OF EGFR MUTATIONAL STATUS IN MATCHED TISSUE AND PLASMA FROM NSCLC PATIENTS TREATED WITH CO-1686
MO21.10 对接受CO-1686的NSCLC患者的血液和组织中的血浆EGFR T790M水平的连续监测和EGFR突变状态的评估
Heather A. Wakelee, Chris A. Karlovich, Wei Wen, Jong-Mu Sun, Sean Chien, Elaina Mann, Patrick O‘Donnell, Philipp Angenendt, Rafal Dziadziusko, Leora Horn, David Spigel, Lecia V. Sequist, Benjamin Solomon, Jean-Charles Soria, D Ross Camidge, Jonathan Goldman, Shirish Gadgeel, Mitch Raponi, Lin Wu, Keunchil Park
Background: We explored the minimally-invasive detection of EGFR mutations in circulating free DNA from plasma and studied the concordance of EGFR mutation status between matched plasma and tumor tissue in a cohort of newly diagnosed or relapsed patients with advanced NSCLC. CO-1686 is an oral, potent, smallmolecule irreversible tyrosine kinase inhibitor that selectively targets mutant forms of EGFR, including T790M and the common initial activating mutations, while sparing wild-type EGFR. Promising clinical activity has recently been reported from an on-going Phase I/II trial.
背景:我们探索了在新诊断出晚期或者复发的NSCLC患者中对血液中游离循环DNA的最小侵入式检测,研究了在对应的血液和肿瘤组织中的EGFR突变状态的一致性。CO-1686是一种口服高效的小分子不可逆TKI,能选择性地靶向突变型的EGFR,包括带有T790M突变以及通常初始激活的突变,同时能避开野生型EGFR。最近来自一项正在进行的Phase I/II期临床已经报告了其有希望的临床活性。
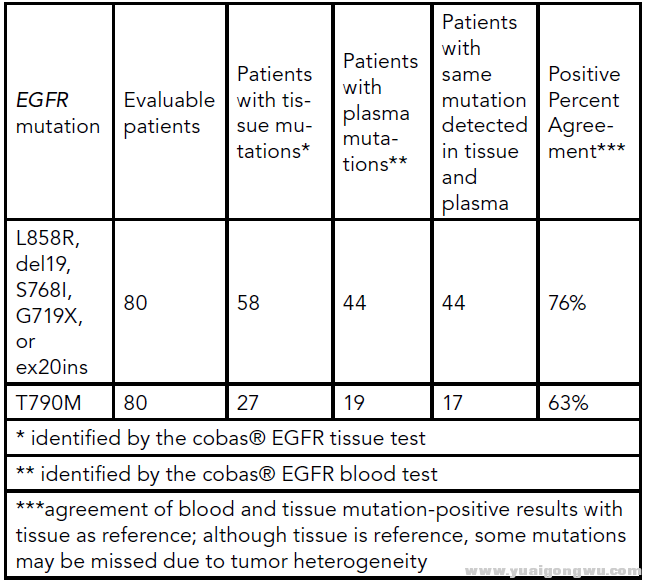
Methods: Matched tumor tissue and blood from 80 Stage IIIB/IV NSCLC patients, 41 treated with CO-1686, were tested using two allele-specific PCR assays, the cobas EGFR FFPET and cobas EGFR blood tests. Each test detects 41 mutations in EGFR, including the T790M resistance mutation, exon 19 deletions and
L858R. We also used BEAMing, a highly quantitative and sensitive technology based on digital PCR, to assess a subset of 18 patients treated with CO-1686. BEAMing was compared to cobas analysis at baseline, and also used to serially monitor plasma EGFR mutation levels in response to CO-1686.
方法:我们采用两种等位基因特异性PCR方法、cobas EGFR FFPET(福尔马林固定石蜡包埋组织)测试和cobas EGFR血液测试,检测来自于80名患者的对应的肿瘤组织和血液样本,其中41位患者已经接受过CO-1686治疗。每项测试检测EGFR的41种突变,包括T790M耐药突变,外显子19缺失突变和L858R错义突变。我们仍然使用基于数字PCR的高量化和敏感性的BEAMing技术,来评估18位接受CO-1686治疗的患者。BEAMing结果将与采用cobas分析的基线数据作对比,同时也被用于对CO-1686治疗响应的血液EGFR突变水平的连续监测。
Results: Using tissue as reference, the positive percent agreement between tissue and plasma was 76% (44/58) for activating mutations and 63% (17/27) for T790M. The cobas EGFR blood test identified two patients with T790M mutations in plasma that were not detected in the corresponding tumor biopsy—likely because of tumor heterogeneity. The M1a/M1b status was known for 63 EGFR mutation-positive patients. Of the 44 with extrathoracic metastatic disease (M1b), 38 were found to have an activating mutation in plasma (86%). Conversely, only 53% (10/19) of EGFR mutation-positive patients with intrathoracic metastatic disease (M1a) had detectable activating mutations in plasma (p = 0.0081). For the 18 patients profiled by BEAMing, the overall percent agreement between BEAMing and the cobas® EGFR blood test was 94% (17/18) for T790M and 83% (15/18) for activating mutations. Nine of the 18 patients had detectable baseline plasma T790M levels, and several patients treated with CO-1686 had an initial decrease in plasma T790M by BEAMing.
结果:使用组织样本做对照,在肿瘤组织和血液样本中的激活突变(19del/L858R)的阳性一致率为76%(44/58),T790M的阳性一致率为63%(12/27)。cobas EGFR血液测试识别出两位患者的血浆中有T790M突变,但由于肿瘤异质性,并未在对应的肿瘤活检样本发现T790M。63位突变患者的分期为已知的M1a/M1b状态。在44位具有胸腔外转移(M1b)的患者中,38位患者被检测出在血浆中具有激活突变(86%)。与此相反,只有53%(10/19)的只含有胸腔内转移(M1a)的患者的血浆中被检测到激活突变(p = 0.0081)。对于采用BEAMing检测的18位患者,BEAMing和cobas EGFR血液检测对于T790M的总一致率为94%(17/18),对于激活突变的总一致率为83%(15/18)。其中9位患者具有可检测的基线T790M血浆水平,部分接受CO-1686治疗的患者表现出对应的血浆T790M水平的下降。
Conclusion: Using the cobas EGFR blood test, a high proportion of EGFR mutations identified in tissue were also detected in plasma. Mutations were more readily detectable in the plasma of patients with M1b rather than M1a disease. These findings suggest that the cobas EGFR blood test and BEAMing can be useful tools for the non-invasive assessment and monitoring of EGFR mutations in NSCLC patients.
总结:在组织样本存在EGFR突变的患者的血液样本中,使用cobas EGFR血液测试能以很高的比例检测到对应的突变。M1b患者具有比M1a患者更高的血液突变检出性。这个发现提示对NSCLC患者来说,cobas EGFR血液测试和BEAMing技术可作为有效的EGFR突变非侵入式评估和监测工具。
感觉这个研究的主要贡献在于能够以更低侵入性的方式检测IV期患者,在接受TKI治疗后的T790M状态,从而更好的指导后续用药。其中提到M1a和M1b的血液和组织的T790M阳性一致率的差别,也是说明即使同为IV期,也需要根据转移灶位置细分为不同的亚组,从而更好的进行针对性的治疗 |